Prostate cancer is the most common cancer among men in the United States with about 1 in 8 men being diagnosed with prostate cancer during his lifetime. It is also one of the leading causes of cancer death among men of all races.1Radiotherapy remains a standard treatment option for prostate cancer with excellent cure rates. However, depending on the kind of radiation, there remains a 10% to 38% rate of published grade 2+ gastrointestinal (GI) acute toxicity associated with prostate radiotherapy.2-6 Symptoms include diarrhea, colic, proctitis and intermittent rectal bleeding. Limiting these side effects associated with radiation exposure to the rectum remains a routine challenge for radiation oncologists due to the close anatomical proximity of the prostate to the rectum.
Rectal toxicity has become even more of a consideration in the current era of moderate and extreme hypofractionated radiotherapy, such as prostate stereotactic body radiotherapy (SBRT). These approaches intend to shorten the radiotherapy course duration; however, they potentially come at the cost of heightened toxicity, especially to the rectum.
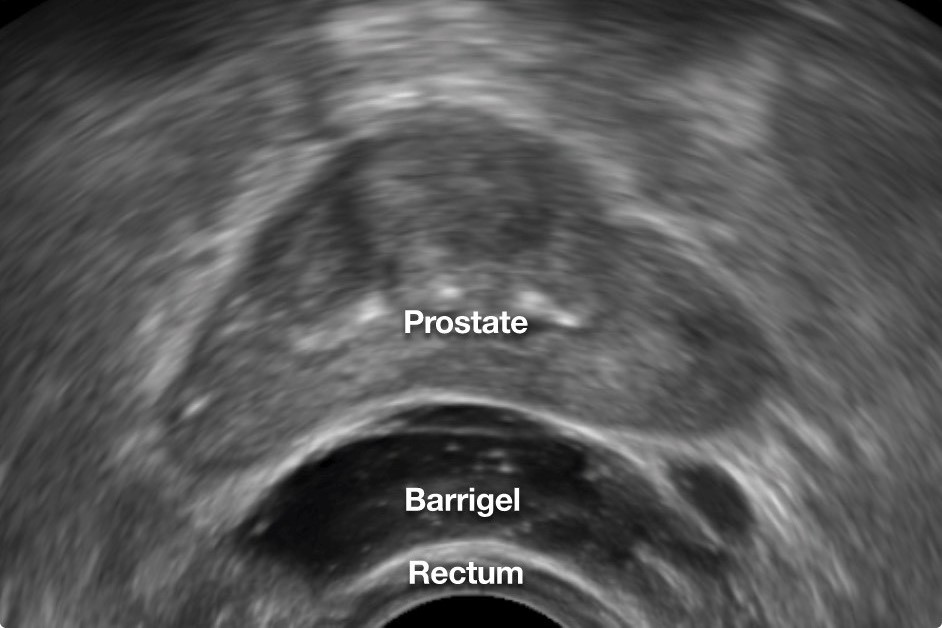
A novel solution to reduce the radiation dose to the anterior rectum is the transperineal placement of a rectal spacer in order to temporarily displace the rectal wall away from the high-dose area of the prostate during a patient’s radiotherapy course.
The first product of its kind to receive U.S. Food and Drug Administration (FDA) approval in 2015 was a polyethylene glycol (PEG) based hydrogel spacer. More recently, Barrigel®, a hyaluronic acid (HA) based rectal spacer, received FDA approval in 2022. In comparison to the PEG hydrogel spacer, the Barrigel spacer offers a controlled spacing environment for safe and effective placement.
Among its advantages, Barrigel offers no injection-time restraints, permitting sculptable control of layering more gel where needed in order to achieve an anatomy-specific custom implant. It also provides clearer visibility on ultrasound during insertion, resulting in greater control and precision of placement. Barrigel additionally affords the opportunity to pause and perform safety checks throughout the procedure. Lastly, this hyaluronic acid spacer offers the option to reverse its application by using hyaluronidase, thereby further enhancing its safety profile.
The procedure to insert the HA spacer can be performed with local, regional or general anesthesia, and can be done as an in-office procedure. NASHA (non-animal stabilized hyaluronic acid) used for Barrigel is a hyaluronic acid preparation produced from non-animal sources, which has been used safely for over 25 years in various medical applications such as treating facial wrinkles, osteoarthritis, vesicoureteral reflux and fecal incontinence.
Published clinical studies in the radiation oncology literature have thus far been encouraging. The Barrigel Prostate Trial, a multinational randomized controlled trial of 201 patients treated with hypofractionated radiation, found that 98.5% of men who were treated with Barrigel met the primary endpoint of achieving at least a 25% reduction in radiation dose to the rectum.7 Patients who met the primary endpoint averaged an 85% reduction in radiation to the rectum. Clinically, the Barrigel spacer arm had a 2.9% rate of acute grade 2 or higher GI toxic effects, reduced from 13.8% for the control group, p=0.01. There were no Barrigel-related adverse events or device-related discomfort.
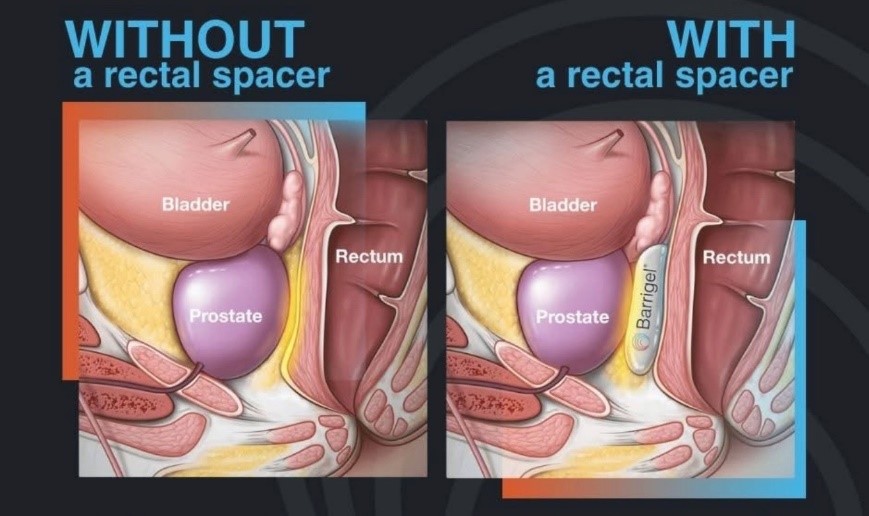
Barrigel injected between the prostate and the rectum supports treating patients with higher radiation doses per treatment using hypofractionated radiotherapy, resulting in less treatment-related rectal side effects for these men. In our experience, we have found the placement of Barrigel spacer to be a safe, easy maneuver to help avoid significant rectal toxicity and preserve bowel QOL without adding significant cost or morbidity to radiation treatment for prostate cancer.
Learn more about prostate cancer treatment at Northside.
References:
- Siegel, R. L., K. D. Miller, H. E. Fuchs, A. Jemal, and others. “Cancer Statistics, 2025.” CA: A Cancer Journal for Clinicians 75, no. 1 (2025): 10–45.
- U.S. Department of Health and Human Services. NCI Common Terminology Criteria for Adverse Events (CTCAE) v5.0. Published November 27, 2017.
- Fransson, P., J. Nilsson, J. Gunnlaugsson, and others. “Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer (HYPO-RT-PC): Patient-reported Quality-of-life Outcomes of a Randomised, Controlled, Non-inferiority, Phase 3 Trial.” Lancet Oncology 22, no. 2 (2021): 235–45.
- Rodda, S., J. Tyldesley, J. Morris, and others. “ASCENDE-RT: An Analysis of Treatment-Related Morbidity for a Randomized Trial Comparing a Low-Dose-Rate Brachytherapy Boost with a Dose-Escalated External Beam Boost for High- and Intermediate-Risk Prostate Cancer.” International Journal of Radiation Oncology, Biology, Physics 98, no. 2 (2017): 286–95.
- Kerkmeier, L. G. W., A. van der Voort van Zyp, I. M. Lips, and others. “Focal Boost to the Intraprostatic Tumor in External Beam Radiotherapy for Patients with Localized Prostate Cancer: Results from the FLAME Randomized Phase III Trial.” Journal of Clinical Oncology 39, no. 7 (2021): 787–96.
- Dearnaley, D., D. Syndikus, C. Mossop, and others. “Conventional versus Hypofractionated High-Dose Intensity-Modulated Radiotherapy for Prostate Cancer: 5-Year Outcomes of the Randomised, Non-inferiority, Phase 3 CHHiP Trial.” Lancet Oncology 17, no. 8 (2016): 1047–60.
- Mariados, N. F., S. P. Sylvester, R. Shah, and others. “Hyaluronic Acid Spacer for Hypofractionated Prostate Radiation Therapy: A Randomized Clinical Trial.” JAMA Oncology 9, no. 4 (2023): 511–18.

